News
MOST RECENT NEWS
-

Merry Christmas and Happy New Year 2025!
-

encevis at the Annual Meeting of American Epilepsy Society in Los Angeles
-

Meet us at the AES2024 meeting in Los Angeles, December 6th-10th!
-

November is epilepsy awareness month
-

encevis integrated into esumedics system
-

encevis V2.1 receives FDA-clearance!
-
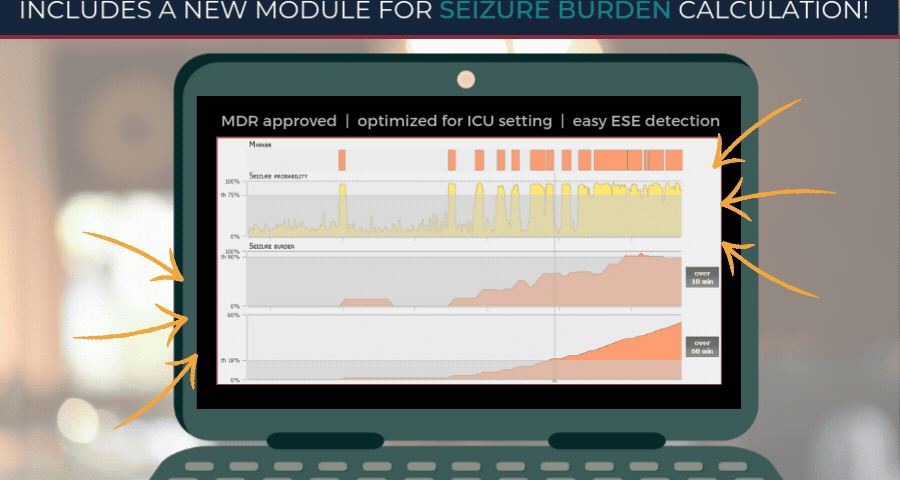
encevis V2.1 released!
-
encevis at European Epilepsy Congress in Rome
-

Meet us at the 15th EEC meeting in Rome, September 7th-11th!
GENERAL NEWS
-
Merry Christmas and Happy New Year 2025!
-
November is epilepsy awareness month
-
encevis integrated into esumedics system
-
encevis integrated in the new Zeto One system
-
Strategy days of our Medical Signal Analysis Group
-
Meet our new colleague – Michael Egger
-
International Epilepsy Day (14.02.2024)
-
Merry Christmas and Happy New Year 2024!
-
encevis on the DIO platform
-
Meet our new colleague – Petra Pudleiner
CONFERENCE NEWS
-
encevis at the Annual Meeting of American Epilepsy Society in Los Angeles
-
Meet us at the AES2024 meeting in Los Angeles, December 6th-10th!
-
encevis at European Epilepsy Congress in Rome
-
Meet us at the 15th EEC meeting in Rome, September 7th-11th!
-
encevis at the annual meeting of the Deutschen Gesellschaft für Epileptologie in Offenburg
-
Meet us at the 62nd DGfE meeting in Offenburg, June 12th-15th!
-
encevis at the dHealth meeting in Vienna – part 2
-
encevis at the dHealth meeting in Vienna – part 1
-
Meet us at the dHealth meeting 2024 in Vienna, May 7th-8th!
-
encevis at London-Innsbruck Colloquium on Status Epilepticus and Acute Seizures in Salzburg
RESERACH & OUTREACH NEWS
-
Presentation of results on automatic seizure burden estimation
-
Article from Austria Presse Agentur (APA) about AI in healthcare
-
Our “encevis4kids” project in GesundheitWirtschaft.at
-
Interview in “Die Presse” about our stroke project
-
encevis strives towards the next milestone!
-
Poster presentation at Conference in Lausanne, October 11-13
-
Poster presentation at IEEE-BSN Conference in Boston, October 9-11
-
Interview in Gesundheitswirtschaft.at about our project on early diagnosis of dementia
-
New publication based on the use of encevis spike detection
-
Our new project on early dementia diagnosis on TV